Meeting USP General Chapter 800 Standards for Hazardous Drugs
Forensic Analytical Consulting Services, Inc. (FACS) was asked to evaluate a company’s compliance with the United States Pharmacopeia (USP) General Chapter 800 standards for hazardous drug (HD) handling. Numerous critical issues were identified to assist the client in achieving compliance.
Key Results
FACS conducted a thorough site visit and documented each non-compliant or risky practice.
Each observation was matched with a recommendation based on regulatory requirements and industry best practices, including OSHA and USP 800 standards
The client was able to implement several process improvements, providing a safer environment for staff and patients and documenting compliance with regulatory standards and guidelines governing their use of hazardous drugs.

About the Client
The client stores, handles, and administers hazardous drugs, primarily for the treatment of cancer. The assessment process applies to any business or institution concerned about compliance with USP 800 standards.

The Problem
The client was concerned about staff safety practices during chemotherapy drug preparation, handling, and administration. This assessment was prompted by the need to ensure compliance with occupational health standards and to optimize staff protection from potential exposures to hazardous drugs.
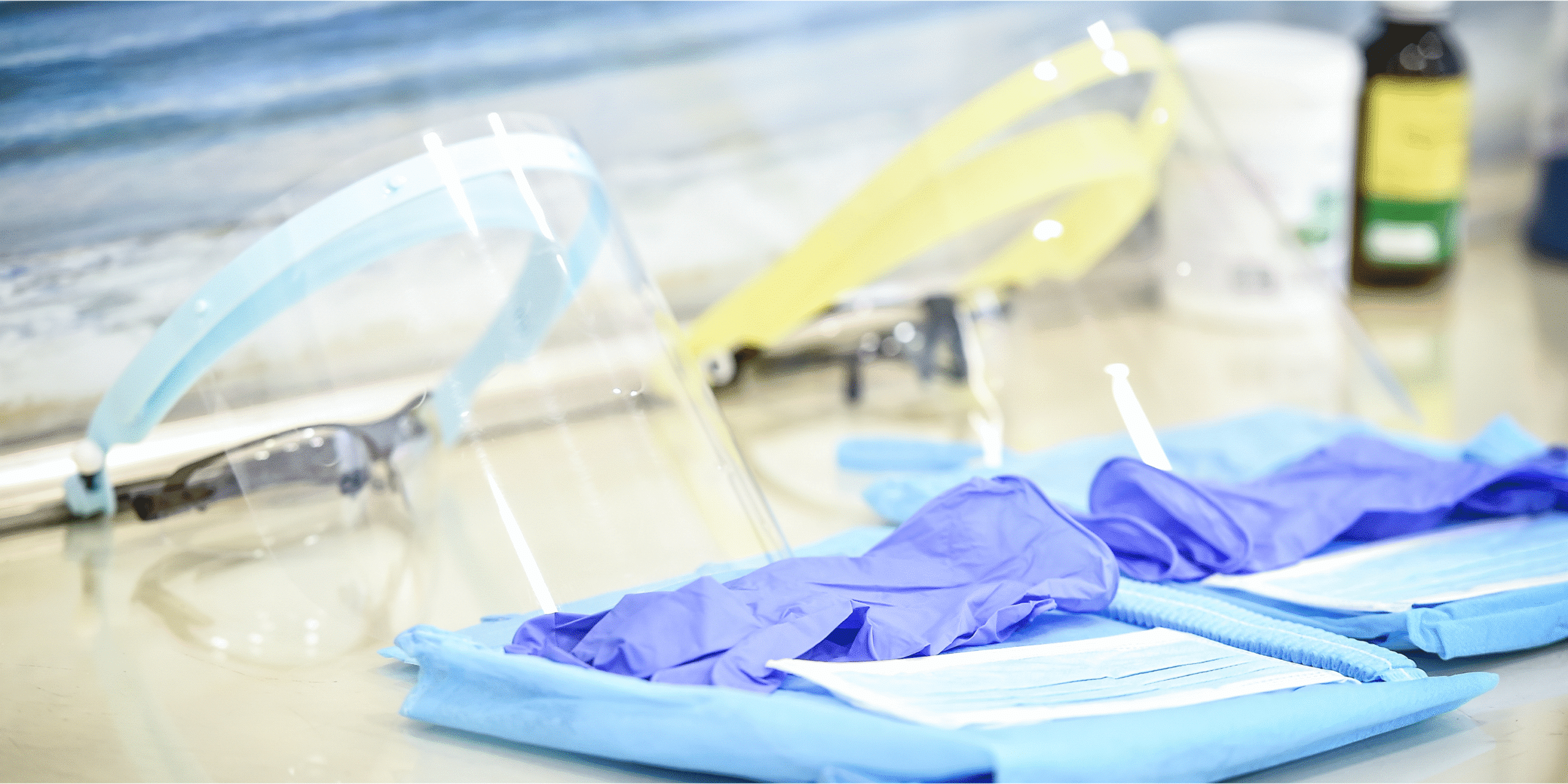
The FACS Solution
FACS’ findings and recommendations provided the client with a clear action plan to optimize staff safety and regulatory compliance. FACS’ guidance emphasized the importance of proper engineering controls, PPE usage, employee training, and facility hygiene in environments where hazardous drugs are stored, handled, and administered. This assessment provided the exact information the client needed to better protect employees and maintain compliance with applicable regulations.